Best Preanalytic Practices
The Centers for Disease Control and Prevention state that 70% of medical decisions depend on laboratory test results.[1] This illustrates the importance and influence of best practice in the preanalytical area on the evaluation in clinical laboratories.
The following are several best practices that should be kept in mind to ensure high quality samples and accurate test results.
Identify the patient
- Minimum requirements include the patient’s full name, date of birth and a patient-specific identifier (e.g. ID card, patient wristband)
- Confirm identity by soliciting verbal response to open-ended questions requiring specific information
- Compare information provided to wristband, which must be attached to the patient, and test requisition
- Resolve any discrepancies prior to proceeding with specimen collection
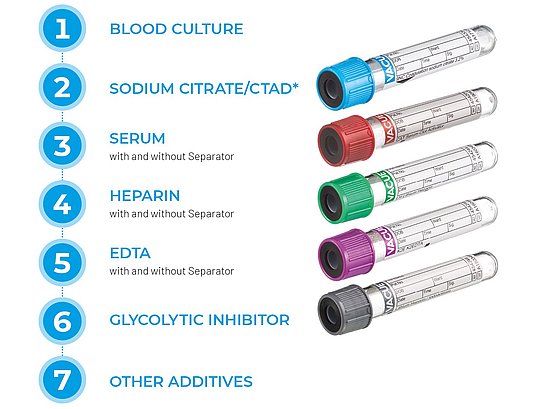
Follow the order of draw
Collect tubes according to the Order of Draw as established in CLSI GP41[2] and according to facility policy.
Preventing under-filled tubes
- Push tube forward so stopper is fully penetrated
- Hold blood collection tube according to IFU and by pressing the tube with the thumb or finger to ensure complete vaccum draw
- Keep tube in place until vacuum is exhausted and blood ceases to flow into tube
- Use discard tube if winged collection set is used and coagulation tube is drawn first
Preventing hemolysis
- Tourniquet application should not be overly constrictive nor should it exceed one minute
- Allow alcohol to dry according to IFU prior to proceeding
- Choose appropriate equipment based on circumstance of the draw
- Fill tubes completely and mix with recommended number of gentle inversions
- If collecting from a vascular access device (VAD), an appropriate transfer device should be used, discard volumes considered and proper procedures for flushing the line should be followed. The discard volume of the VAD is required due to particles from the puncturing process.
- Avoid excessive pulling force when using a syringe and follow proper procedure for transfer to evacuated tubes following collection
Preventing fibrin formation
- Fill tubes to indicated level to ensure proper blood to additive ratio
- Gently invert tubes according to IFU to mix the additive with the sample
- Allow serum tubes to clot thoroughly (minimum 30 minutes) in an upright position
Label tubes
- Label tubes in presence of patient
- Reconfirm patient identification
- Place the patient label along the top edge of the tube label such that additive and fill mark remain visible
There may be additional considerations depending on the patient condition and the tests ordered. Another helpful article for more information can be found at https://www.gbo.com/en-us/spotlight/article/the-top-5-phlebotomy-basics-to-know.
[1]Division of Laboratory Systems (DLS). Strengthening Clinical Laboratories. 2018. https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html
[2]Clinical and Laboratory Standards Institute (CLSI). Collection of Diagnostic Venous Blood Specimens. 7th ed. CLSI standard GP41 (ISBN 1-56238-812-6 [Print]; ISBN 1-56238-813-4 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, 2017.
[3] VACUETTE® Best Preanalytic Practices. Greiner Bio-One North America, Inc.
This product information is addressed exclusively to healthcare professionals. Devices of Greiner Bio-One are to be used by properly trained healthcare professionals only in accordance with the relevant Instructions for Use (IFU). For a listing of indications, contraindications, precautions and warnings, please refer to the Instructions for Use which accompanies each product or is available for download from our website at www.gbo.com (Download Center). For more information contact your local Greiner Bio-One sales representative or visit our website.
All information is provided without guarantee despite careful processing. Any liability, warranty or guarantee of Greiner Bio-One GmbH is excluded. All rights, errors and changes are reserved. If not stated otherwise, Greiner Bio-One GmbH has all copyrights and/or other (user-)rights in this documents, in particular to signs such as the mentioned (word-picture-)brands and logos. Any use, duplication or any other use of the rights of Greiner Bio-One GmbH is expressly prohibited.
Media owner and publisher: Greiner Bio-One GmbH, Bad Haller Str. 32, 4550 Kremsmünster